- What Is Multimodal AI in Telehealth?
- Core Features of Multimodal AI Telehealth Platforms
- How Multimodal AI Works in Telehealth Systems
- Clinical Applications of Multimodal AI in Telehealth
- Key Benefits of Multimodal AI in Telehealth Development
- Challenges in Multimodal AI Telehealth Development
- HIPAA Compliance for Multimodal AI in Telehealth
- Development Process for Multimodal AI Telehealth Platforms
- Multimodal AI Telehealth Platform Development Cost
- Partner with Space-O AI for Building Your Multimodal Telehealth Solution
- Frequently Asked Questions on Multimodal AI Telehealth Development
- 1. What is multimodal AI in telehealth?
- 2. How does multimodal AI improve diagnostic accuracy?
- 3. What data types can multimodal telehealth AI process?
- 4. Is multimodal AI HIPAA compliant?
- 5. How long does multimodal AI telehealth development take?
- 6. What is the ROI of multimodal AI in telehealth?
- 7. Can multimodal AI integrate with existing telehealth platforms?
Multimodal AI in Telehealth Development: Building Intelligent Multimodal Care Systems
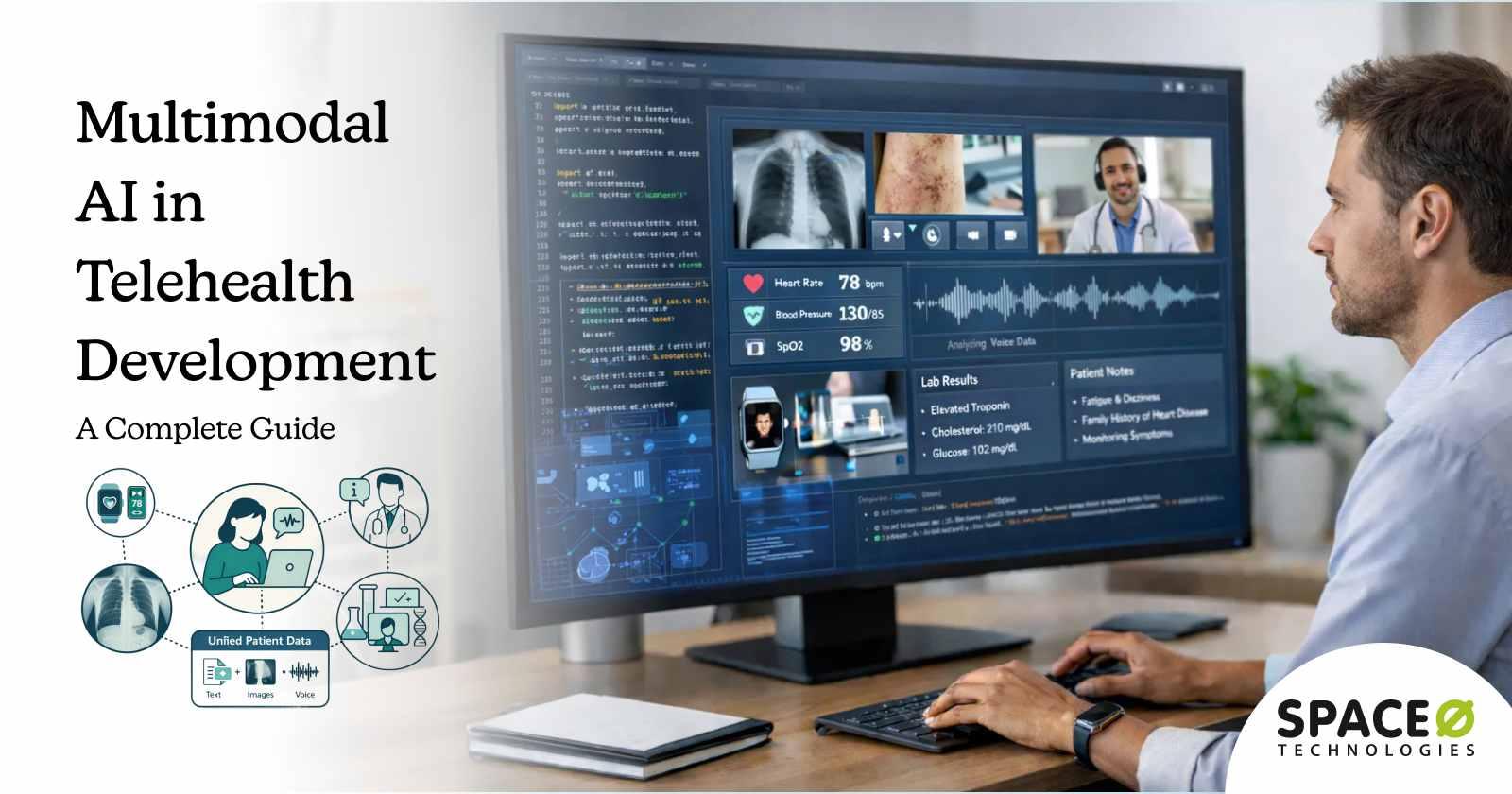
Telehealth has moved from being a convenience to an expected standard of care. According to the National Telehealth Survey, 89% of Americans stated they were fully satisfied with their telehealth appointments, highlighting strong patient acceptance of virtual healthcare delivery.
As telehealth adoption grows, patient expectations are also evolving. Users now expect virtual care experiences that feel as responsive, personalized, and clinically effective as in-person visits. Traditional telehealth platforms, however, often rely on isolated data inputs such as video calls or text-based interactions, limiting clinical context and decision-making.
This is where multimodal AI is transforming telehealth development. By enabling telehealth solutions to simultaneously interpret text, voice, images, video, and structured health data, multimodal AI creates a more complete patient view. This allows healthcare organizations to improve clinical accuracy, streamline workflows, and deliver more engaging virtual care experiences.
In this blog, we explore how to develop a telehealth platform with multimodal AI features, key use cases across virtual care, and strategic development considerations. Get insights from our 15+ years of experience as a leading AI telehealth development partner to understand how to build secure, scalable, and patient-centric multimodal telehealth solutions.
What Is Multimodal AI in Telehealth?
Multimodal AI in telehealth refers to artificial intelligence systems designed to process, analyze, and correlate multiple types of healthcare data simultaneously. Unlike traditional AI that specializes in one data type, multimodal systems create unified patient representations by combining insights from diverse sources.
Think of how an experienced physician approaches diagnosis. They do not rely solely on an X-ray or just the patient’s verbal description. They synthesize imaging results, lab values, patient history, physical observations, and even subtle cues like voice tone and facial expressions. Multimodal AI replicates this holistic approach computationally.
The distinction matters because healthcare data is inherently multimodal. A patient’s health status cannot be fully captured by any single data source. Chest pain might appear normal on an ECG but be concerning when combined with elevated troponin levels, family history, and stress indicators from wearable data. This approach to multi-modal medical data analysis catches correlations that single-modality AI completely misses.
Unified patient data processing, the core concept behind multimodal telehealth AI, means creating a comprehensive digital representation of each patient that updates in real-time as new data arrives from any source. This enables diagnostic insights impossible with fragmented systems.
Data modalities in multimodal telehealth AI
Modern multimodal telehealth platforms can process seven primary data categories, each contributing unique diagnostic value.
1. Medical imaging data
This modality includes X-rays, CT scans, MRI results, ultrasounds, and dermatology photographs. These visual inputs provide structural and anatomical information essential for many diagnoses.
2. Electronic health records
EHRs contain clinical notes, medication lists, allergies, past diagnoses, and treatment histories. This text-based data provides longitudinal context no other modality offers.
3. Vital signs and time-series data
This category encompasses heart rate, blood pressure, oxygen saturation, temperature, and respiratory rate. Continuous monitoring reveals patterns invisible in spot measurements.
4. Audio and voice data
Voice modality captures patient consultations, symptom descriptions, and speech patterns. Voice analysis can detect respiratory conditions, neurological changes, and mental health indicators.
5. Video data
Video feeds from telemedicine consultations enable visual assessment of patient appearance, movement, skin condition, and non-verbal cues that text descriptions miss.
6. Wearable sensor data
Wearables provide continuous streams from smartwatches, glucose monitors, sleep trackers, and activity sensors. This data captures health patterns between clinical encounters.
7. Genomic and molecular data
Genetic data offers risk factors, pharmacogenomic profiles, and biomarker information that personalizes diagnosis and treatment recommendations.
| Pro Tip: Start with 2-3 data modalities that your organization already collects reliably. Expanding to additional modalities is easier once your fusion architecture is proven. Trying to integrate all seven modalities at once is a common reason multimodal projects fail. |
Understanding these modalities sets the foundation for designing systems that leverage them effectively. The next critical consideration is what features your multimodal platform needs to deliver clinical value.
Core Features of Multimodal AI Telehealth Platforms
Building a multimodal AI telehealth platform requires specific technical capabilities. The features you prioritize determine whether your system delivers real clinical value or becomes another underutilized tool. Based on our experience delivering AI software development services for healthcare organizations, these twelve features form the foundation of successful multimodal implementations.
1. Data ingestion and preprocessing features
These features handle how your platform collects and prepares data from multiple sources before analysis begins.
1.1 Multi-source data connectors
This feature enables real-time ingestion from EHR systems, imaging archives using PACS, wearable device APIs, and video streaming platforms. Robust connectors support healthcare standards, including HL7 FHIR, DICOM, and custom proprietary formats for seamless data integration across your technology ecosystem.
1.2 Modality-specific preprocessing pipelines
These pipelines handle the unique requirements of each data type automatically. Medical images undergo artifact removal and normalization. Clinical text passes through specialized healthcare NLP for entity extraction. Vital signs are cleaned, interpolated, and time-aligned before fusion.
1.3 Missing data handling engine
This engine addresses the reality that patient records are rarely complete. Intelligent imputation uses cross-modal inference to estimate missing values while flagging uncertainty levels. This ensures your AI functions reliably even with incomplete data.
2. Fusion and analysis features
These features determine how your platform combines and analyzes data from multiple modalities to generate insights.
2.1 Cross-modal fusion engine
The fusion engine combines extracted features from multiple data sources using attention mechanisms that learn which modalities matter most for specific clinical questions. Configurable fusion strategies support early, late, and hybrid approaches depending on use case requirements.
2.2 Temporal alignment module
This module synchronizes time-series data arriving from different sources at different intervals. Wearable data sampled every second must align with lab results from yesterday and imaging from last week. Proper temporal alignment creates accurate longitudinal patient timelines.
2.3 Real-time inference capability
Real-time inference processes multimodal data during live telemedicine consultations, delivering diagnostic insights within seconds. Latency optimization ensures AI recommendations arrive while they are still clinically relevant, not after the consultation ends.
3. Clinical decision support features
These features translate multimodal analysis into actionable clinical insights that help physicians make better decisions.
3.1 Differential diagnosis generator
The diagnosis generator analyzes combined multimodal data to produce ranked diagnostic possibilities. Each suggestion includes confidence scores and specific evidence from each modality, showing clinicians exactly why the AI reached its conclusions.
3.2 Risk stratification dashboard
This dashboard visualizes patient risk levels derived from multimodal analysis. Interactive displays highlight which data sources contributed most to risk calculations, enabling clinicians to focus attention on the most concerning indicators.
3.3 Explainable AI outputs
Explainability features provide transparent reasoning for all recommendations. When your AI suggests a diagnosis, clinicians see exactly which image regions, text phrases, vital patterns, or sensor readings influenced that conclusion. Explainability builds trust and supports clinical documentation.
4. Integration and compliance features
These features ensure your multimodal AI platform works within existing healthcare infrastructure while meeting regulatory requirements.
4.1 EHR and telehealth platform connectors
Platform connectors provide pre-built integrations with major systems, including Epic, Cerner, Teladoc, and Amwell. Bidirectional data flow ensures multimodal insights appear within existing clinical workflows without requiring clinicians to learn new interfaces.
4.2 HIPAA-compliant data handling
Compliance features implement end-to-end encryption, role-based access controls, comprehensive audit logging, and automatic PHI de-identification for training datasets. Compliance is built into the architecture rather than bolted on afterward.
4.3 MLOps and model monitoring
MLOps capabilities enable continuous performance tracking after deployment. Automated data drift detection identifies when real-world patterns diverge from training data, triggering alerts and retraining workflows to maintain diagnostic accuracy over time.
Feature prioritization by implementation phase
The following table summarizes feature priorities based on the implementation phase.
| Feature Category | Must-Have for MVP | Add for Production | Enterprise Advanced |
| Data Ingestion | Multi-source connectors, basic preprocessing | Real-time streaming, quality scoring | Edge processing, federated ingestion |
| Fusion Engine | Late fusion, temporal alignment | Hybrid fusion, attention mechanisms | Graph neural networks, cross-modal transformers |
| Clinical Support | Diagnosis generation, basic risk scores | Explainable AI, confidence intervals | Uncertainty quantification, counterfactual explanations |
| Compliance | Encryption, access controls, and audit logs | Automated de-identification, BAA management | Federated learning, on-premise deployment options |
These features require skilled implementation. Organizations often hire AI developers with healthcare experience to ensure clinical requirements translate correctly into technical specifications.
With features defined, understanding how these components work together architecturally becomes essential for making informed technology decisions.
How Multimodal AI Works in Telehealth Systems
Multimodal AI follows a six-step pipeline from data ingestion to clinical delivery. Here is how each stage works.
Step 1: Data collection
The system ingests data from multiple sources simultaneously: EHR systems (clinical notes, medications, history), PACS servers (medical images), wearable devices (real-time vitals), video conferencing (consultation footage), and patient portals (self-reported symptoms).
Step 2: Preprocessing
Each data type undergoes specialized cleaning. Images are normalized and quality-scored. Clinical text passes through NLP to extract medical entities. Vital signs are cleaned of sensor dropouts. Audio converts to transcripts with speaker separation. Video extracts facial landmarks and movement data.
Step 3: Feature extraction
Preprocessed data is converted into numerical embeddings. CNNs encode images. Transformers process clinical text. Recurrent networks handle time-series vitals. Each encoder produces compact representations preserving diagnostically relevant information.
Step 4: Multimodal fusion
This critical step combines modality embeddings into unified representations. Fusion strategy choice balances accuracy against complexity.
| Fusion Method | Complexity | Best For |
| Early Fusion | High | Research environments |
| Late Fusion | Low | Production MVP, regulated settings |
| Hybrid Fusion | Medium | Mature production systems |
| Attention-based | Highest | Complex diagnostics |
| Pro Tip: Late fusion is the safest starting point. It lets you validate each modality’s model independently before combining them, reducing debugging complexity and regulatory risk. |
Step 5: Clinical inference
Fused representations feed into task-specific models generating: diagnostic predictions with confidence scores, risk stratification with contributing factors, treatment recommendations with evidence, and alert triggers for urgent findings.
Step 6: Workflow delivery
Results reach clinicians through real-time video overlays, EHR integration at decision points, mobile notifications for urgent findings, and population health dashboards. Timing and placement determine whether clinicians adopt AI recommendations.
Understanding these architectural options helps when working with AI development teams to design systems that match your requirements.
These technical foundations enable the clinical applications that deliver actual patient value. The next section explores where multimodal AI creates the most impact in telehealth settings.
Clinical Applications of Multimodal AI in Telehealth
Multimodal AI transforms telehealth capabilities across multiple clinical domains. Understanding specific applications helps organizations prioritize development efforts toward the highest-impact use cases.
1. Remote diagnostic accuracy improvement
Combining multiple data sources dramatically improves diagnostic precision in virtual care settings.
1.1 Differential diagnosis with comprehensive data
This application enables AI to consider imaging findings alongside patient history, current medications, and real-time vitals. A chest X-ray analyzed in isolation might appear normal, but combined with elevated inflammatory markers, recent travel history, and slightly decreased oxygen saturation, the same image supports a different diagnostic conclusion.
1.2 Cancer subtype prediction
This use case combines imaging with genomic data and clinical characteristics. Multimodal analysis can distinguish between cancer subtypes that appear identical on imaging alone, guiding treatment selection without requiring additional invasive procedures.
1.3 Cardiovascular risk assessment
This application integrates ECG patterns, echocardiogram findings, lab results including lipid panels and inflammatory markers, blood pressure trends from home monitoring, and activity data from wearables. This comprehensive view predicts cardiac events more accurately than any single data source.
1.4 Dermatology diagnosis
This capability combines high-resolution skin images with patient-reported symptom duration, medication history, family history of skin conditions, and affected body area patterns. Multimodal analysis distinguishes between visually similar conditions that require different treatments.
The diagnostic applications above require AI specialists who can build models for medical imaging, clinical text analysis, and vital sign interpretation. Organizations building these capabilities partner with experienced AI development teams to ensure each data modality receives appropriate technical expertise.
2. Personalized treatment planning
Multimodal AI enables treatment recommendations tailored to individual patient characteristics.
2.1 Drug response prediction
This application combines genetic pharmacogenomic profiles with current medication lists, lab values indicating organ function, and historical response patterns. This predicts which medications will work best for each patient while minimizing adverse reaction risk.
2.2 Chronic disease management protocols
These protocols adapt based on continuous multimodal monitoring. A diabetes management plan adjusts in real-time based on glucose sensor data, dietary logs, activity levels, stress indicators, and medication adherence patterns.
2.3 Dosage optimization
This capability uses patient weight, organ function labs, genetic metabolism markers, and real-time response monitoring to recommend personalized dosing. This is particularly valuable for medications with narrow therapeutic windows.
3. Enhanced virtual consultations
Real-time multimodal analysis during telemedicine visits augments clinician capabilities.
3.1 Simultaneous video and vital analysis
This feature processes patient appearance, movement, and expressions while monitoring connected device data. Subtle discrepancies between what patients say and what their vitals show become visible to clinicians.
3.2 Mental health assessment
This application combines facial micro-expression analysis, voice tone and speech pattern evaluation, and patient-reported outcomes. Multimodal analysis detects depression, anxiety, and cognitive changes earlier than questionnaires alone.
3.3 Physical therapy monitoring
This capability uses video-based movement analysis combined with wearable motion sensor data and patient-reported pain levels. Therapists see objective progress metrics alongside subjective reports during virtual follow-ups.
| Pro Tip: Mental health telehealth shows the highest ROI for multimodal AI. Combining voice tone analysis, facial micro-expressions, and patient-reported outcomes can detect depression relapse 2–3 weeks earlier than traditional assessments alone. |
These applications leverage AI-powered analysis capabilities, combining visual, audio, and text processing for comprehensive consultation support.
4. Remote patient monitoring
Continuous multimodal monitoring extends clinical oversight between visits.
4.1 Early warning systems
These systems combine wearable vital signs, activity patterns, sleep quality, and symptom reports to predict clinical deterioration before it becomes emergent. Alerts notify care teams when multimodal patterns suggest intervention is needed.
4.2 Post-discharge monitoring
This application tracks surgical patients through connected devices, symptom surveys, and medication adherence data. Multimodal analysis identifies patients at risk for readmission while intervention is still possible.
4.3 Chronic disease surveillance
This capability monitors conditions like heart failure, COPD, and diabetes using continuous data streams to detect subtle changes. Weight trends, activity levels, oxygen saturation patterns, and self-reported symptoms together reveal decompensation earlier than any single measure.
These applications deliver measurable value, but realizing that value requires understanding the benefits multimodal AI provides to different stakeholders.
Ready to Build Advanced Multimodal AI for Your Telehealth Platform?
Our healthcare AI specialists design and develop custom multimodal systems that integrate with your existing telemedicine infrastructure seamlessly and securely.
Key Benefits of Multimodal AI in Telehealth Development
Investing in integrated healthcare data AI capabilities delivers advantages across clinical, operational, and competitive dimensions. Each benefit addresses specific pain points that healthcare organizations face with current telehealth systems.
1. Higher diagnostic accuracy
Cross-modal correlation catches what single-source AI misses. Multimodal approaches significantly reduce misdiagnosis rates compared to analyzing each data type separately. Patterns invisible in isolation become clear when imaging, labs, and vitals align together.
2. Comprehensive patient profiling
Multimodal systems create 360-degree views by synthesizing fragmented health data automatically. Clinicians see complete pictures without manually piecing together information from multiple systems, tabs, and reports during time-pressured consultations.
3. Earlier disease detection
Multimodal analysis identifies subtle warning signs across modalities before conditions become symptomatic or severe. Early detection of conditions like diabetic retinopathy, cardiac arrhythmias, and mental health deterioration improves outcomes while reducing treatment costs.
4. Reduced clinician cognitive load
Automation handles the mentally exhausting work of correlating data from different sources. Physicians spend time on patient interaction and clinical judgment instead of toggling between screens, remembering lab values, and cross-referencing medication lists.
5. Improved patient outcomes
Personalized treatment recommendations based on multi-source analysis lead to better results. Better-matched treatments lead to higher adherence, faster recovery, fewer adverse events, and reduced hospital readmissions for chronic disease patients.
6. Operational efficiency gains
Multimodal platforms streamline diagnostic workflows from hours to minutes in many cases. One multimodal analysis replaces multiple single-purpose AI tools, reducing integration complexity, licensing costs, and the training burden on clinical staff.
7. Competitive market differentiation
Advanced capabilities position your telehealth platform above basic video visit offerings. Patients and referring physicians choose platforms demonstrating superior clinical intelligence, creating sustainable competitive advantage in crowded markets.
8. Scalable specialist access
Multimodal AI enables primary care providers to deliver specialist-level insights with AI support. This addresses physician shortages in rural areas and underserved markets where specialist telehealth availability remains limited.
Building these capabilities requires experienced AI teams who understand both the technical complexity of multimodal architectures and the healthcare domain knowledge needed to ensure outputs are clinically relevant and actionable. One must consider partnering with an expert AI consulting service provider for the best results.
Benefits are compelling, but realistic implementation requires understanding the challenges involved and how to address them.
Challenges in Multimodal AI Telehealth Development
Building production-ready multimodal AI systems involves significant technical and organizational hurdles. Acknowledging these challenges upfront enables better planning and more realistic timelines.
1. Data heterogeneity and standardization
Healthcare data exists in wildly different formats across sources. Medical images use DICOM with varying metadata conventions. Clinical notes are unstructured text with institution-specific abbreviations. Vital signs arrive as time-series streams with different sampling rates. Lab results follow inconsistent naming conventions across providers.
Unifying these for AI processing requires substantial engineering effort before any model development begins.
Solution
- Implement HL7 FHIR as the interoperability backbone for structured data exchange
- Build modality-specific preprocessing pipelines with standardized output formats
- Create data quality scoring to flag problematic inputs before they corrupt the analysis
- Use feature alignment layers that map heterogeneous inputs to common representations
- Establish data governance policies defining acceptable formats and quality thresholds
2. Missing and incomplete data handling
Real-world patient records are rarely complete. A patient may have recent imaging but no genetic data. Wearable data contains gaps from device removal or connectivity issues. Historical records from previous providers may be inaccessible. Multimodal AI must function reliably even when modalities are partially or fully missing.
Solution
- Design graceful degradation that provides value with whatever data is available
- Apply cross-modal imputation using learned correlations between modalities
- Implement uncertainty quantification that increases when data is missing
- Train models on realistic data availability patterns matching production conditions
- Build fallback pathways that route to appropriate single-modality analysis when needed
3. Temporal alignment across modalities
Data from different sources arrive at different times with different timestamps and different clinical relevance windows. A chest X-ray from Tuesday, vital signs from Wednesday, and lab results from Friday must be properly aligned to represent a coherent clinical state. Misalignment leads to incorrect correlations and flawed conclusions.
Solution
- Build temporal synchronization pipelines with configurable alignment windows
- Use attention mechanisms that learn temporal relevance for each clinical question
- Implement real-time fusion for live consultation scenarios requiring immediate results
- Define clear data freshness policies specifying acceptable age for each modality
- Handle timezone and timestamp format inconsistencies across source systems
4. Computational requirements and latency
Processing multiple data streams simultaneously demands significant computing resources. Image analysis alone requires substantial GPU capacity. Adding real-time video processing, continuous vital monitoring, and NLP for clinical notes multiplies requirements. For live telehealth consultations, inference must be completed in seconds, creating hard architectural constraints.
Solution
- Deploy edge computing for latency-sensitive modalities like video analysis
- Use model optimization techniques, including quantization, pruning, and knowledge distillation
- Implement cloud-edge hybrid architectures, balancing capability with responsiveness
- Design asynchronous processing pipelines for analyses that can tolerate delay
- Right-size infrastructure based on realistic concurrent usage patterns
5. Model explainability and clinical trust
Clinicians will not adopt AI recommendations they cannot understand or verify. Multimodal systems present unique explainability challenges because outputs depend on complex interactions between data types. Explaining why a diagnosis changed based on the combination of an image finding, a lab value, and a vital sign trend requires sophisticated interpretability techniques.
Black-box multimodal models face resistance in clinical settings where physicians bear legal and ethical responsibility for decisions. Regulatory bodies increasingly require explainability for AI medical devices.
Solution
- Implement attention visualization showing which modalities and features influenced each prediction
- Provide counterfactual explanations describing what would change the output
- Generate natural language summaries of reasoning accessible to non-technical clinicians
- Build confidence calibration so that stated certainty reflects actual accuracy
- Design human-in-the-loop workflows where AI augments rather than replaces clinical judgment
| Pro Tip: Build your multimodal system to work in “degraded mode” from day one. If the imaging server is down, your AI should still provide value from available text and vitals. This resilience is often what separates production systems from failed pilots. |
Addressing technical challenges is necessary but insufficient. Regulatory compliance adds another layer of complexity that cannot be overlooked.
Overcome Multimodal AI Development Challenges with Expert Guidance Today
Our team has solved complex healthcare AI integration challenges for hospitals and telehealth startups worldwide. Let us help you navigate technical hurdles efficiently.
HIPAA Compliance for Multimodal AI in Telehealth
Multimodal AI systems face heightened compliance requirements because they access and process more PHI types than single-modality solutions, making HIPAA-compliant telemedicine developmentap critical. Every additional data source expands the protected information surface area requiring safeguards.
Compliance checklist for multimodal telehealth AI
Before diving into specific requirements, use this checklist to assess your compliance posture.
| Requirement | Standard | Implementation Priority |
| Encryption at rest | AES-256 for all modalities | Critical |
| Encryption in transit | TLS 1.3 or higher | Critical |
| Access controls | Role-based with MFA | Critical |
| Audit logging | All PHI access and AI predictions | Critical |
| Data minimization | Process only required PHI | High |
| Business Associate Agreements | All vendors touching PHI | Critical |
| De-identification | Safe Harbor or Expert Determination | High |
| Incident response | 60-day breach notification | Critical |
| Risk assessment | Annual multimodal-specific evaluation | High |
Why multimodal systems require extra vigilance
The fundamental challenge is that multimodal systems correlate data in ways that can inadvertently re-identify patients even from supposedly anonymized datasets. Combining imaging metadata, treatment patterns, and demographic information can uniquely identify individuals even when direct identifiers are removed.
1. PHI protection requirements
Understanding what constitutes protected information in each data type is essential for compliance.
1.1 PHI in medical imaging
Medical images contain embedded patient identifiers in DICOM headers. Metadata includes patient names, dates of birth, medical record numbers, and facility information that must be stripped or encrypted.
1.2 PHI in voice and video
Voice recordings capture biometric identifiers that can identify individuals. Video consultations show faces, which constitute biometric PHI. Both require special handling and consent considerations.
1.3 PHI in wearable data
Even anonymized wearable data patterns can be identified when combined with other information. Activity patterns, sleep schedules, and location data create unique fingerprints.
2. Technical safeguards
Implementing proper technical controls protects PHI throughout the multimodal data lifecycle.
2.1 Encryption requirements
Data must be encrypted at rest using AES-256 or equivalent standards, as outlined in the HHS HIPAA Security Rule. All transmissions must use TLS 1.3 or higher. Encryption keys require secure management with rotation policies and access controls separate from encrypted data.
2.2 Access control implementation
Access controls must follow the minimum necessary principle. Role-based permissions ensure clinicians access only data relevant to their patient relationships. Technical controls prevent unauthorized bulk data extraction. Multi-factor authentication is mandatory for all PHI access.
2.3 Audit trail requirements
Audit trails must capture all data access, model predictions, and user actions in tamper-evident logs. When AI makes a recommendation, the audit trail must record what data inputs contributed, enabling investigation of any adverse outcomes.
3. Administrative safeguards
Policies and procedures complement technical controls.
3.1 Business Associate Agreements (BAAs)
BAAs are required with every vendor touching PHI, including cloud providers, AI model vendors, and integration partners. BAAs must specifically address AI and machine learning use cases, including training data handling.
3.2 De-identification for model training
De-identification requires careful application of Safe Harbor or Expert Determination methods. Multimodal data is particularly challenging because re-identification risks increase when multiple data types are combined. Conservative approaches remove more information than might seem necessary.
| Pro Tip: Keep training data and inference data in separate environments with different access controls. Many HIPAA violations occur when de-identified training data gets accidentally linked back to production PHI through shared infrastructure. |
Compliance establishes the guardrails. Within those guardrails, a structured development process maximizes the chance of successful implementation.
Development Process for Multimodal AI Telehealth Platforms
Building multimodal AI for telehealth requires disciplined execution across five phases. Each phase has specific deliverables that must be completed before proceeding, reducing the risk of costly rework later.
Step 1: Define clinical objectives and data requirements
Start by identifying specific clinical outcomes you want to improve. Vague goals like “better diagnostics” lead to unfocused development and unclear success criteria. Precise objectives like “reduce diabetic retinopathy screening false negatives by 30%” provide clear direction and measurable targets.
This phase determines everything that follows. Objectives misaligned with available data or organizational capabilities doom projects before development begins.
Action items
- Identify 2–3 measurable clinical KPIs that multimodal AI should impact
- Map required data modalities for each objective with the current availability assessment
- Conduct a data quality audit across all candidate modalities
- Define success metrics and minimum performance thresholds before development starts
- Secure clinical stakeholder alignment on objectives and evaluation criteria
Step 2: Design multimodal data architecture
Architecture decisions made during this phase determine long-term scalability, maintainability, and regulatory compliance posture. Choosing fusion strategies, data pipelines, and integration patterns requires balancing clinical objectives against technical constraints and organizational capabilities.
Invest adequate time in architectural design. Rebuilding data pipelines mid-project is expensive and demoralizing.
Action items
- Select a fusion approach based on use case requirements and data characteristics
- Design modality-specific preprocessing pipelines with standardized interfaces
- Plan integration points with existing EHR, PACS, and telehealth systems
- Define data governance policies, including retention, access, and quality standards
- Document architecture decisions and rationale for future maintenance teams
Step 3: Build and train multimodal models
Model development requires diverse, representative training data across all modalities. Bias in any single data source propagates through the entire multimodal system, making data quality and representativeness paramount. Validation must cover diverse patient populations to ensure equitable performance.
Action items
- Prepare balanced training datasets with proper clinical annotations and quality verification
- Implement cross-modal learning using architectures appropriate for your fusion strategy
- Validate model performance across demographic groups to identify bias
- Conduct a clinical review of model outputs with domain experts
- Document model development decisions for regulatory submissions if applicable
Step 4: Integrate with telehealth workflows
Integration determines whether clinicians actually use your multimodal AI. Systems that add clicks, require context switching, or slow consultations get abandoned regardless of underlying accuracy. Design for seamless embedding within existing clinical workflows.
Action items
- Connect to video consultation platforms via APIs that enable real-time data flow
- Enable inference during virtual visits with latency appropriate for clinical use
- Build clinician-facing interfaces that fit existing workflow patterns
- Implement feedback mechanisms allowing clinicians to correct AI errors
- Train clinical staff on interpreting and acting on multimodal AI outputs
Step 5: Deploy, monitor, and optimize
Production deployment marks the beginning of operational responsibility, not the end of development. Healthcare data patterns shift as patient populations change, new treatments emerge, and clinical practices evolve. Without monitoring and maintenance, model performance degrades.
Action items
- Implement MLOps pipelines for continuous performance monitoring
- Track both technical metrics and clinical outcome measures
- Set up automated alerts for performance degradation or data drift
- Establish model retraining schedules based on observed drift patterns
- Plan capacity for ongoing model updates and feature additions
This structured process delivers production-ready systems, but organizations need realistic budget expectations before committing resources.
Multimodal AI Telehealth Platform Development Cost
Developing a production-ready multimodal AI telehealth platform typically costs between $150,000 and $500,000 or more. The table below breaks down AI telemedicine development costs by implementation complexity.
| Complexity Level | Data Modalities | Key Features | Timeline | Estimated Cost Range |
| Basic MVP | 2–3 modalities | Late fusion, basic diagnostics, cloud deployment | 4–6 months | $150,000–$250,000 |
| Intermediate | 4–5 modalities | Hybrid fusion, real-time processing, EHR integration | 6–9 months | $250,000–$400,000 |
| Enterprise | 6+ modalities | Attention-based fusion, explainable AI, FDA-ready | 9–14 months | $400,000–$500,000+ |
Most organizations start with a Basic MVP to validate clinical value before scaling. The jump from basic to intermediate reflects the added complexity of real-time processing and deeper EHR integration. Enterprise builds include regulatory preparation and advanced explainability features required for clinical adoption.
Key cost drivers
1. Number of data modalities
Each modality (e.g., audio, video, clinical images, vitals from devices, natural language text, biosignals) requires its own preprocessing, model components, annotation/validation data pipelines, and integration logic. More modalities → higher engineering effort, model complexity, and QA needs.
2. Real-time vs. batch processing
Real-time inference (e.g., live consultation, diagnostic support) requires low-latency models, optimized serving infrastructure, and additional testing. Batch processing (e.g., overnight analytics) is technically simpler and cheaper to operate.
3. Integration depth
- Surface API integration (basic data exchange) is lower cost.
- Deep bidirectional workflows with EHR/EMR systems, clinical decision support, billing, scheduling, and provider documentation require significant engineering and interoperability (FHIR/HL7) work.
4. Regulatory pathway
Platforms that incorporate clinical decision support, diagnosis, or predictions may be subject to medical device regulation (e.g., FDA SaMD, CE marking), requiring documentation, clinical validation, quality management systems, and post-market surveillance. FDA/CE pathways can add tens of thousands to hundreds of thousands to costs.
5. Compliance and security
HIPAA, GDPR, and other data protection standards are essential and add implementation, auditing, and ongoing assessment costs.
Ongoing operational costs
Beyond initial development, budget for these recurring expenses:
- Infrastructure: $5,000-$25,000 monthly for cloud computing based on usage patterns
- Maintenance: 15-20% of initial development cost annually for MLOps, monitoring, and model improvements
- Regulatory compliance: $50,000-$150,000 for HIPAA audits, penetration testing, and FDA submissions
- Training: $20,000-$50,000 for clinical staff onboarding and change management
| Pro Tip: Start with a 2-modality MVP to prove clinical value before expanding. A successful $150K pilot that demonstrates 30% diagnostic improvement is far more valuable than a $500K system that never reaches production. |
Get an Accurate Cost Estimate for Your Multimodal AI Project
We provide detailed project scoping and transparent cost breakdowns tailored to your specific clinical objectives and technical requirements.
Partner with Space-O AI for Building Your Multimodal Telehealth Solution
Multimodal AI in telehealth development transforms virtual care by unifying imaging, text, voice, and sensor data into actionable clinical intelligence. From enhanced diagnostic accuracy to personalized treatment recommendations, this technology addresses fundamental limitations of fragmented single-source AI systems.
Space-O AI brings 15+ years of software development expertise to healthcare AI challenges. Our team understands both the technical complexity of multimodal architectures and the regulatory requirements essential for production-ready telehealth platforms.
With 80+ developers experienced in machine learning, computer vision, NLP, and healthcare system integrations, we have delivered HIPAA-compliant AI solutions for hospitals, specialty clinics, and telehealth startups. Our multimodal implementations operate reliably in production environments serving real patients.
Ready to build multimodal AI capabilities into your telehealth platform? Schedule a free consultation with our healthcare AI specialists today. We will assess your specific requirements and outline a clear path from initial concept to successful production deployment.
Frequently Asked Questions on Multimodal AI Telehealth Development
1. What is multimodal AI in telehealth?
Multimodal AI in telehealth refers to artificial intelligence systems that analyze multiple data types simultaneously, including medical images, clinical notes, voice recordings, video feeds, and wearable sensor data. Unlike single-modality AI that processes one data type, multimodal systems correlate patterns across diverse sources to generate more accurate and comprehensive diagnostic insights.
2. How does multimodal AI improve diagnostic accuracy?
Multimodal AI improves diagnostic accuracy by detecting patterns that exist only when multiple data sources are analyzed together. For example, combining imaging results with vital sign trends and medication history reveals correlations invisible when analyzing each source separately. Research indicates multimodal approaches reduce misdiagnosis rates by 40-60% compared to single-source analysis.
3. What data types can multimodal telehealth AI process?
Multimodal telehealth AI can process medical imaging such as X-rays, CT scans, and MRI, electronic health records containing clinical notes and medication lists, real-time vital signs, audio from patient consultations, video feeds from telemedicine visits, continuous data from wearable devices, and genomic or molecular information. Specific modalities depend on platform design and clinical objectives.
4. Is multimodal AI HIPAA compliant?
Multimodal AI can achieve HIPAA compliance when properly architected with end-to-end encryption, role-based access controls, comprehensive audit logging, and appropriate Business Associate Agreements covering all vendors. The multi-source nature requires particular attention to PHI protection across all data types and careful de-identification procedures for any training data.
5. How long does multimodal AI telehealth development take?
Development timelines range from 4 to 6 months for a basic MVP incorporating two to three data modalities to 9 to 14 months for enterprise systems with six or more modalities and FDA-ready documentation. Timelines depend on data availability and quality, integration complexity with existing systems, fusion architecture sophistication, and regulatory requirements.
6. What is the ROI of multimodal AI in telehealth?
Return on investment comes from improved diagnostic accuracy that reduces costly misdiagnoses and malpractice exposure, operational efficiency gains from automated data correlation, reduced clinician burnout and improved retention, and competitive differentiation that attracts patients and referrals. Organizations typically achieve a 30-50% reduction in diagnostic errors and 40% faster clinical decision-making.
7. Can multimodal AI integrate with existing telehealth platforms?
Yes, multimodal AI integrates with existing telehealth platforms through APIs and standard healthcare interoperability protocols such as HL7 FHIR for clinical data and DICOM for imaging. Pre-built connectors for major EHR systems like Epic and Cerner, and telehealth platforms such as Teladoc and Amwell, enable integration without replacing existing infrastructure.
Build Smarter Telehealth with Multimodal AI
What to read next



